Category
Health policy
-
Report shows rising insurance costs erode workers’ earnings, increase disparities
Rising health insurance premium rates have cost families with workplace coverage more than $125,000 in earnings over three recent decades,…

-
Coverage 101: Using the new state media guides to write big stories
In this webinar, learn how to navigate and report on health insurance in each state with Georgetown University’s new primers.

-
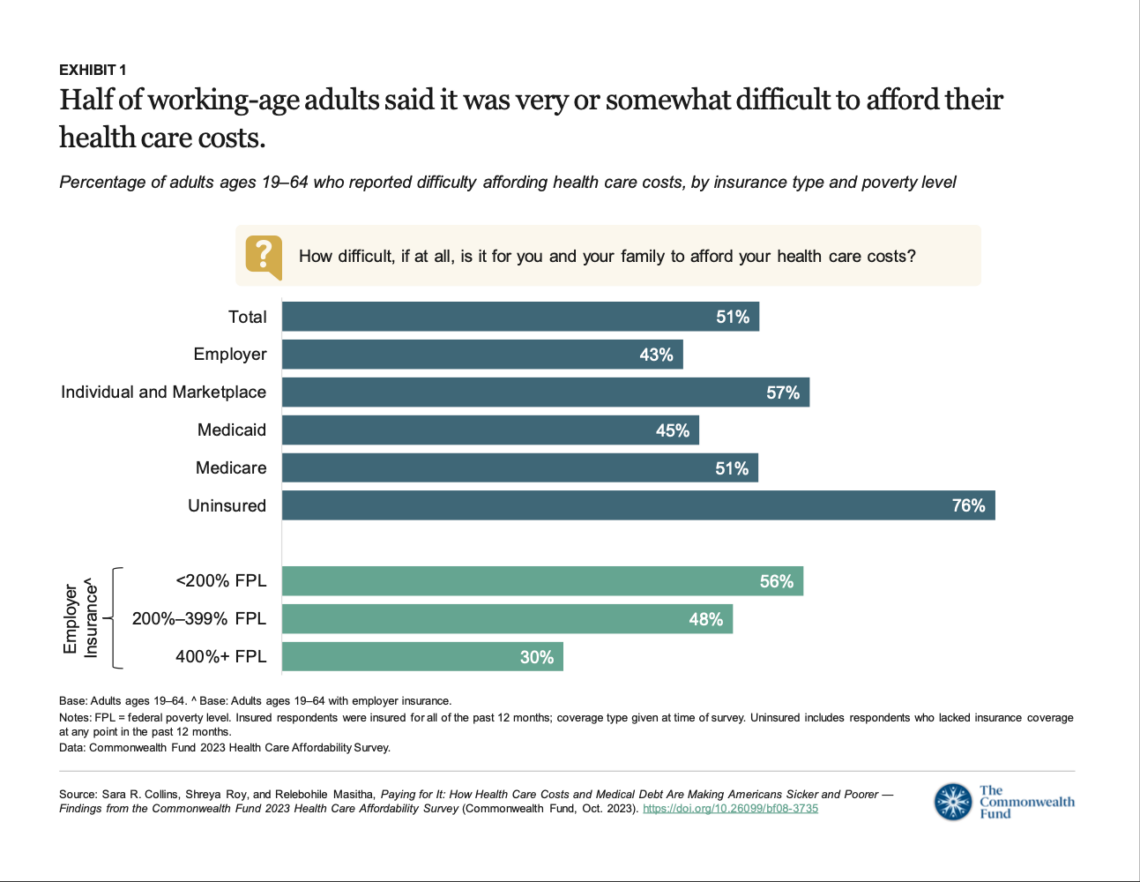
Report shows health insurance coverage is inadequate for many Americans
Health insurance provides insufficient coverage for the care most Americans need, a recent survey shows.

-
With Roe likely in its final days, experts say reporters should sharpen focus on abortion as a health issue
Pregnancy is a medical condition and abortion is an intervention for it, so journalists writing about the topic should take…

-
FDA Commissioner Califf sounds the alarm on health misinformation
Food and Drug Administration (FDA) Commissioner Robert Califf, M.D., M.A.C.C., isn’t easy to rattle. During a Q&A on Friday, April…

-
Public records: Dig deep (but curb your expectations)
Lexi Churchill of ProPublica (at the podium) and Sandhya Kambhampati of the Los Angeles Times (to the right) talking to…

-
Becerra needs to open up to the press
AHCJ is calling on Health and Human Services Secretary Xavier Becerra to make himself available for questioning by reporters. In…

-
Tracking the Senate confirmation process for next FDA chief
The Senate’s vetting of a proposed Food and Drug Administration (FDA) commissioner may provide an opportunity for reporters to dig…

-
Who is Jeffrey Zients, the soon-to-be White House coronavirus czar?
As President-elect Joe Biden develops a strategy for ending the pandemic, the person who will be in charge of executing…

-
SCOTUS ruling allows states to regulate what PBMs pay pharmacists
States seeking to regulate pharmacy benefit managers (PBMs) won an important victory on Thursday when the U.S. Supreme Court ruled…