Tag
vaccine
-
Reporting responsibly on COVID-19 vaccines requires knowing the research landscape
A new New York Times perspective piece on whether we’re underselling the various COVID-19 vaccines had public health Twitter abuzz…

-
Major COVID-19 vaccine ad campaign to roll out next week
Journalists covering the COVID-19 vaccine rollout should watch out on Jan. 21 for the Ad Council’s unveiling of an advertising…

-
Pfizer vaccine news sounds great — but it’s still data by press release
Pfizer made waves Monday with its announcement that its COVID-19 vaccine, developed with partner BioNTech, is “strongly effective,” with a…

-
Get prepared now to cover the COVID-19 vaccine
Photo: Photo: VCU Capital News Service via Flickr The race for a COVID-19 is heating up. At least two COVID-19…

-
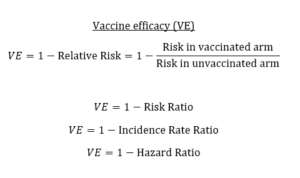
Know the nuances of vaccine efficacy when covering COVID-19 vaccine trials
I’ve written in previous posts about what to look for in COVID-19 vaccine trials and red flags to monitor. The…

-
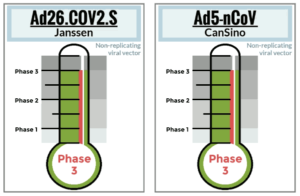
Tracker a one-stop-shop for monitoring COVID-19 vaccine development
It’s been a dizzying task for reporters trying to keep up with the development of COVID-19 vaccines. There are the…

-
Follow COVID-19 research money to find stories near you
The timeless advice sometimes attributed to Deep Throat or Bob Woodard and Carl Bernstein to “follow the money” certainly applies…

-
What to look for in COVID-19 vaccine trials
As various COVID-19 vaccine candidates make their way through clinical trials — see this nice update on where things stand…

-
CDC highlights case study on how schools could reopen safely
To showcase how schools could reopen safely this year, Centers for Disease Control and Prevention director Robert Redfield, M.D., highlighted…

-
COVID-19 plus flu season could overwhelm health care system
As we start moving into fall and winter, Americans are certain to be facing a continuation of COVID-19, the disease…