Tag
fda
-
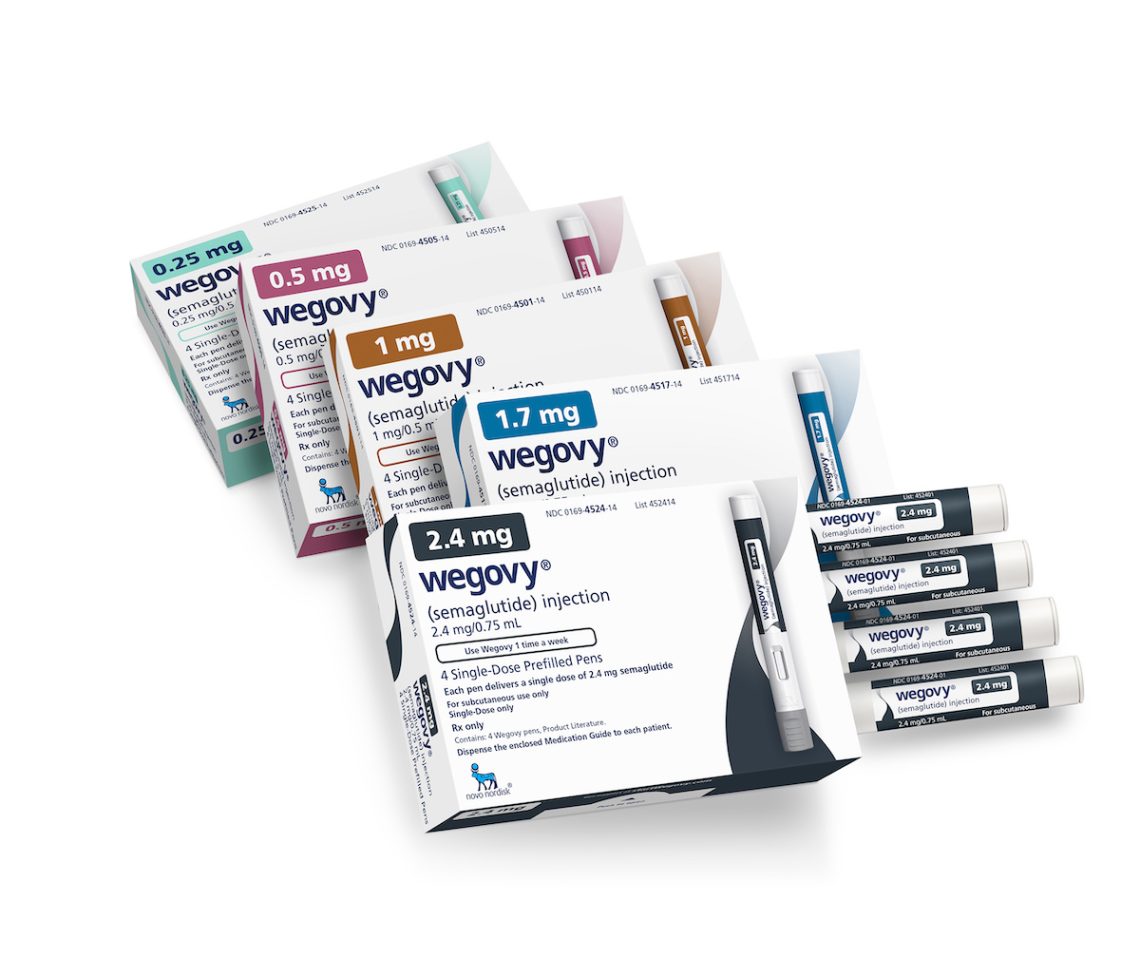
How to cover the sharp rise in spending for diabetes and weight loss drugs
Consumer demand for expensive diabetes and weight loss medications like Ozempic could drive up insurance premiums and Medicare costs.

-
Why journalists should scrutinize the FDA’s accelerated drug approval process
Despite recent reforms, journalists still need to be diligent about covering the evidence on which accelerated approvals are often based.

-
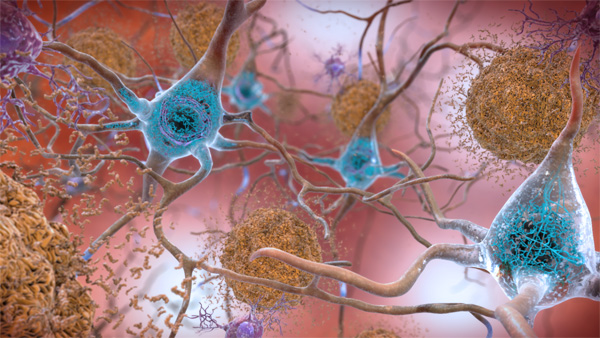
Q&A: What to know about new Alzheimer’s drug Leqembi
The FDA granted full clinical approval to Leqembi (lecanemab) on Thursday, July 6, making it only the second new drug…

-
6 prescription digital therapeutics story angles to explore
The news that Highmark, a Pittsburgh-based large commercial insurer, plans to expand its coverage of several prescription digital therapeutics cleared by the…

-
Evaluating concerns about accelerated FDA drug approvals
Congress has a chance in September to try to speed the pace of studies needed to confirm whether drugs sold…

-
Rise in naloxone costs for uninsured, a significant access barrier to life-saving drug
The cost of naloxone has risen sharply for people lacking insurance, even as laws have made it easier to prescribe…

-
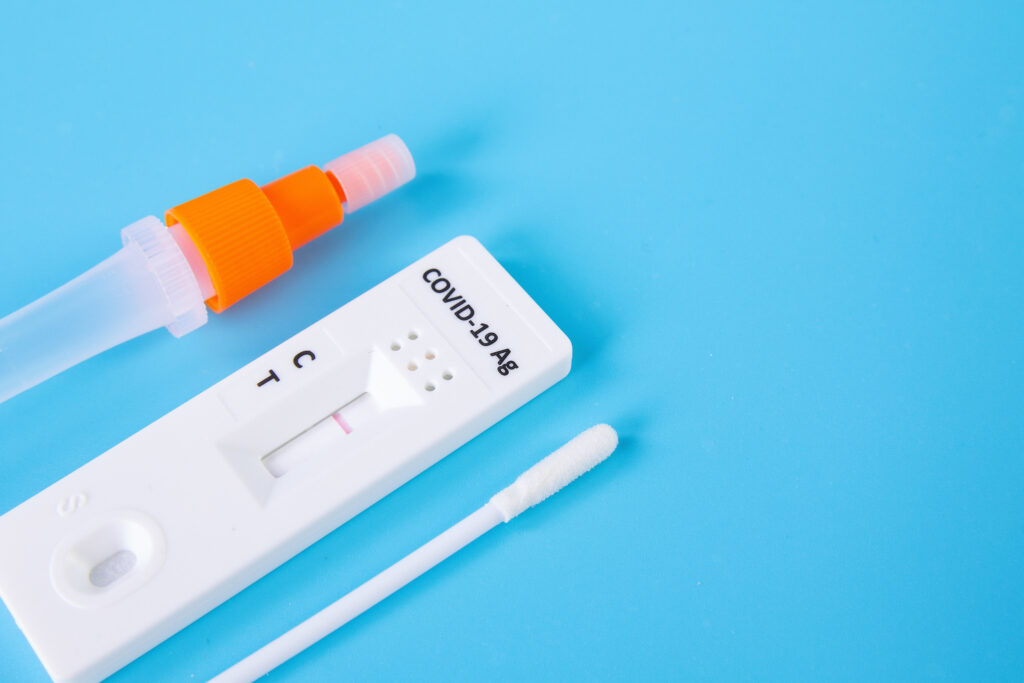
How to use COVID-19 rapid tests to avoid misleading results
Rapid antigen tests are now the standard way for people to determine if they have COVID-19, but studies show they…

-
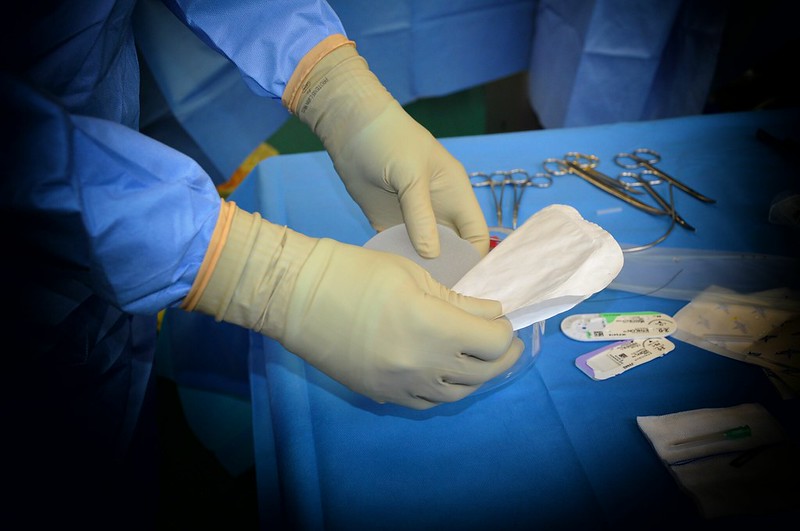
FDA warns that breast implants are not “lifetime devices”: Tips for covering this story
The Food and Drug Administration (FDA) announced last week a series of steps meant to address long-standing concerns about breast…

-
Alzheimer’s drug approved Monday by FDA raises questions for journalists
Part two of two parts; the first ran Thursday, June 10. There’s still a great deal we don’t yet understand…

-
FDA approves new Alzheimer’s drug, but controversy persists
Part one of two parts; the second runs tomorrow, Friday, June 11. You might think that the first new drug…