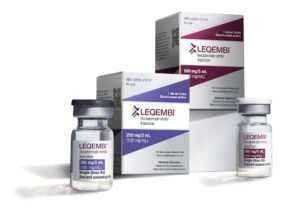
The FDA granted full clinical approval to Leqembi (lecanemab) on Thursday, July 6, making it only the second new drug approved to fight Alzheimer’s disease and the first to receive full authorization from the agency.
The approval follows unanimous endorsement of the drug’s safety, efficacy and clinical benefit by an independent advisory committee in June. The anti-amyloid drug had already received accelerated approval in January 2023. Full FDA authorization is a big step for potentially thousands of people with mild to moderate Alzheimer’s, who may be eligible for treatment.
Leqembi targets and reduces amyloid beta plaque in the brain, which slows progression of the disease. The Centers for Medicare and Medicaid has previously said it would pay for the drug — at a cost of $26,500 per year — upon full authorization.
Right now, Medicare will only cover the cost of the drug for those participating in clinical trials. Prescribing physicians will be required to participate in a patient registry to help Medicare gather more data about safety and efficacy. It’s also not clear yet whether, or how much, Medicare will pay for the cost of related screening, either via lumbar puncture or PET scan, or how much it will pay for the cost of infusion services, which is the mode of delivery for the drug.
This is a significant milestone in Alzheimer’s treatment, according to Judith Heidebrink, M.D., M.S., a neurologist and clinical core co-lead of the Michigan Alzheimer’s Disease Center. In this Q&A, Heidebrink answers some key questions about the drug, the disease, and what journalists should focus on. Heidebrink was involved in the open label extension of this phase II trial as well as the prevention trial, which is enrolling participants. (The University of Michigan was also involved in the double-blind portion.)
(Answers have been edited for length and clarity).
Why is Leqembi such a big deal for people with Alzheimer’s?
We’ve had therapies to help slow down the progression, but those are the kind of catch-up therapies, helping to rebalance chemical changes that have been there after the nerves are wearing out and people are losing their abilities. We haven’t had therapies that directly target the underlying pathology like the plaque and tangle buildup. Not only does this drug reduce the amyloid buildup in people with early symptoms of Alzheimer’s, but it also slows the clinical progression. So there’s convincing evidence of a clinical benefit. It’s not a cure but a step in the right direction in directly targeting the pathology of the disease and helping people to maintain their independence longer.
Clinical data show a moderate benefit in people taking this drug vs. placebo. Can you explain what that means in the real world?
There was a 27% difference on our primary outcome measure, or 0.45 difference on the sum of boxes on a clinical dementia rating scale. The best way to try to explain it is if you look at the endpoint after 18 months of therapy, the folks on treatment were functioning and behaving the way the placebo group was at roughly the 12-month point. So it slows it (disease progression) down by six months over an 18-month period.
Numerically that would be a third; our outcome measures weren’t quite at that one-third, but this ballparks what we’re talking about. For some people, an extra six months — I can do this on my own for six months longer, I could do that on my own — may be incredibly worthwhile. Someone else may say, I haven’t changed much in the last six months. So is it worth six extra months of where I’ve been, versus coming in every two weeks for an infusion, or dealing with side effects? That might not add up for them. It’s going to be an individual decision.
Can you talk more about those side effects? Several people died after taking the drug.
Most people had a reaction at the infusion site, which fortunately tended to be mild, and could be relatively easily managed. The more serious but, fortunately, less common side effects are essentially a reaction to the drug’s mechanism of action when you’re pulling amyloid out of the brain. Some people may have more amyloid in particular around blood vessels and those blood vessels may leak, so fluid can go into the brain and cause swelling. Or blood can leak out and you can have hemorrhages. Roughly 20% of people in the phase three trial experienced that edema or hemorrhage, but most of the time it was clinically silent, so a much smaller percentage actually had symptoms. But you had to monitor it very carefully. If you did see early signs of it, depending on where and how much, the drug might need to be halted temporarily or sometimes permanently because that could have led to more serious side effects.
There were a few cases, not in the placebo-controlled trial, but in the open label extension, of hemorrhages where people died, but they were in complicated cases where people were receiving a clot-busting drug for a stroke, or a very strong blood thinner. So that gives us pause and we would likely not recommend treatment for people who have these other strong risk factors for bleeding in the brain. We also know genetically, the same major risk factor for Alzheimer’s disease, the APOE lipoprotein E4 Gene (APOE4) also determines whether someone’s at much higher risk of these bleeding or swelling side effects.
Who would be a good candidate for this drug?
In the study, people had to meet certain criteria in terms of their cognitive scores, their daily functioning had to be within a certain range, but they also had had some evidence that amyloid is building up in the brain either typically through things like a PET scan, or spinal fluid markers that are highly predictive of what’s going on in the brain. A spinal tap is not convenient or friendly. And a PET scan right now is not covered by any insurance, and that’s several thousand dollars. Also, there aren’t enough specialists available to evaluate everyone with Alzheimer’s symptoms.
So there are financial barriers — it probably translates to $100,000 a year when you talk about the actual infusion part of it, the MRI monitoring everything else, and if this is all under outpatient services, you’ve got 20% co-pays on all of that. There are logistical barriers — do you live close to a place that has a PET scanner or an MRI scanner and infusion scanner? One of the concerns is not only the health equity issue but what will this do eventually to Medicare premiums (ICER analyses suggest Lecanemab would achieve cost-effectiveness if priced between $8,900 – $21,500 per year.)?
How can health journalists report this story and other Alzheimer’s research responsibly?
I think we have to get the message out that we have to be cautious about any very early experimental research. It just has to work its way through the standard phases of clinical trials to really know if it ultimately is going to pan out, even with drugs like Lecanemab. Again, it’s a foot in the door, not a cure. And it’s only for people who are in the very early stages.
I also think those numbers aren’t going to mean anything to the average person in that clinical dementia rating scale. It’s more helpful to say, in a time sense, how much did it slow it down? Because then we can compare things across trials of other drugs because not all studies will use the same outcome measure or use the same population.
Resources
- This tip sheet will help journalists better understand the differences and similarities between Alzheimer’s disease and other major forms of dementia, as well as a list of resources for these progressive cognitive conditions.
- Here are 4 tips for reporting on press releases for new drugs
- With other Alzheimer’s drugs in the pipeline, here’s what to know about FDA’s accelerated approval process.
- STAT’s reporting on safety concerns about this new drug.