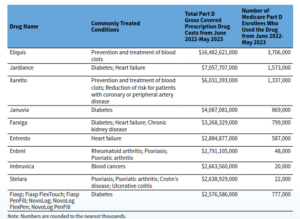
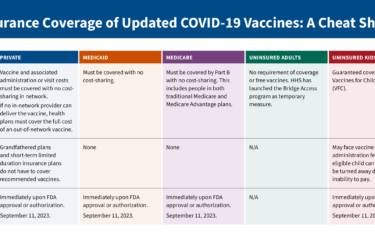
When people carp about the high prices of drugs, the poster kids are often the biotech wonders that can cost tens of thousands of dollars a year.
Sure, a nifty new cancer medicine may be worth a lot of money to the person whose life it lengthens. But should the maker of drugs like those enjoy a price monopoly indefinitely?
For biotech drugs – proteins produced by living cells – the answer in the United States has pretty much been yes because there’s no simple path for the Food and Drug Administration to approve generic versions of blockbusters such as Genentech’s Herceptin (annual cost: $48,000) or Johnson & Johnson’s Remicade (annual cost: $20,000).
As part of Health Policy, Congress is wrestling with rules to clear the way for so-called follow-on biologics. The main sticking point: how long should the inventor of a biotech medicine be protected from rivals?
The Wall Street Journal’s Alicia Mundy reports that the biotech industry appears to be getting its way, with extra protection that could last as long as 13½ years.
The lengthy protection period is being championed by the Senate health maven Ted Kennedy (D-Mass.) and is nearly twice as long as the seven years proposed by the White House. Others in Congress, including Sen. Chuck Schumer (D-N.Y.), have pushed for 5½ years.
Why does the number matter so much? Competition. The Federal Trade Commission concluded in a recent report that protection lasting 12 to 14 years “is unnecessary to promote innovation” and would inhibit the introduction of more biotech options at lower prices.
Update
In a win for the biotech industry, the Senate’s Health, Education, Labor and Pensions Committee voted 16-7 to protect biotech medicines from generic competition for 12 years. The term could still be changed by the full Senate or in negotiations with the House.