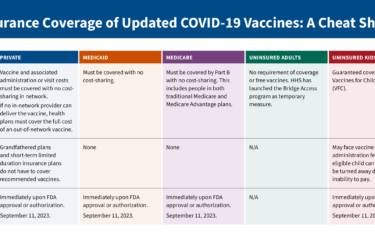
Care to know more about what it takes to accurately weigh medical evidence? You’ve got just a couple of days to jump in to apply for AHCJ’s Comparative Effectiveness Research Fellowship – the deadline is Friday.
Care to know more about what it takes to accurately weigh medical evidence? You’ve got just a couple of days to jump in to apply for AHCJ’s Comparative Effectiveness Research Fellowship – the deadline is Friday.
To get an idea of what you’ll walk away with by joining the select group of health journalists headed to Bethesda, Md., Oct. 7-11, read what former CER fellows said:
Karen Bouffard of the Detroit News: “This was one of the best fellowships I have attended. It provided a good foundation of knowledge of the various ways clinical trials are structured, and the pros and cons of each approach. We learned about the ethical considerations in clinical research, and how these have evolved over time. I left the fellowship with several story ideas and new potential sources.”
Shannon Firth of MedPage Today: “I came away with more in-depth perspectives on some of the most significant discussions in health right now – mammography screening, the high cost of certain drugs and finding the most efficient and ethical way to conduct research whose results can be used in the real world.”
Laura Joszt of the American Journal of Managed Care: “I gained new insight into how to view and assess trials, new story ideas. And learned new ways of viewing research to improve my reporting and how I question the results.”
Sarah Owermohle of S&P Global Market Intelligence: “This fellowship shows how to truly assess the strengths and weaknesses of clinical studies, the ways that they are evolving and how best to represent them as you talk to your audience.”
For this fellowship, AHCJ has teamed up with the Patient-Centered Outcomes Research Institute to guide fellows in learning:
- How to evaluate treatments and how medical decisions are made
- Determine what constitutes reliable research methodology
- Learn how research findings are applied in shared-decision making
- See how comparing treatments could reshape the health system
- Identify the evidence gaps in treating common conditions
- Understand how patient health care data is used in research
- See how health literacy impacts patient outcomes
- Understand coming changes in clinical trials.
For more information on who qualifies, go here.
To apply, go here. Deadline to apply is Aug. 3, noon, CT