If you are familiar with Drugs@FDA, you know that the website allows you to quickly look up a drug by its name (brand), the active ingredient (generic), or application number. But if you frequently work on the go or need to look up something quickly while away from your computer, you now can download the FDA’s new app, Drugs@FDA Express (iOS/Apple and Android/Google), to see much of the same information.
If you are familiar with Drugs@FDA, you know that the website allows you to quickly look up a drug by its name (brand), the active ingredient (generic), or application number. But if you frequently work on the go or need to look up something quickly while away from your computer, you now can download the FDA’s new app, Drugs@FDA Express (iOS/Apple and Android/Google), to see much of the same information.
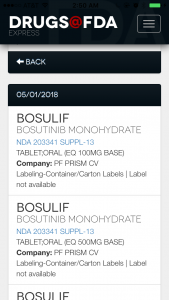
Released in late March, the app is pretty basic, but often that’s the best kind of app. It loads quickly, isn’t overly cluttered and has simpler user-friendly interface. The opening page is straightforward.
For journalists, the “Last 7 Days’ Approval,” organized in reverse date order, is particularly helpful, especially if you missed a press release or are not subscribed to all FDA press releases on new drug approvals.
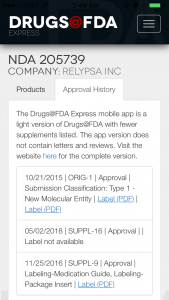
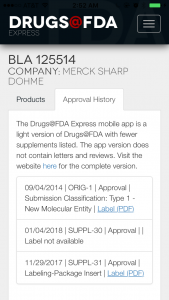
When you click on the drug entry, you have the option to look at its approval history as well. It’s not super detailed, but there’s PDF link that gives the same PDF label you’d see on the website. From there you can download the files via your preferred PDF or document storage app (Evernote, Dropbox, Google Drive, etc.).
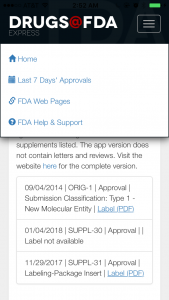
One thing a little non-intuitive about the interface is that its “Back” button is at the bottom instead of the top, so you have to scroll down to go back if there are multiple entries.
However, the upper right hamburger menu will take you to Home or one of the major sections.
Learning about this app led me to look for other FDA apps and I found five total. Most are designed for pharmacists or health care professionals, but I was pleasantly surprised to see an FDA Drug Shortages app, which can be very useful for a journalist covering shortages or checking whether a shortage might be occurring due to a recent outbreak or another event.
The app also loads very quickly and has a simple, clean opening interface.
You can look at drug shortages alphabetically or according to the clinical area.

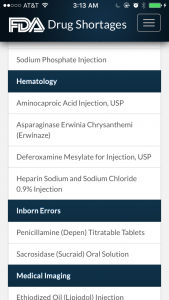
 Again, the entries are basic but provide the most important information: the clinical area the drug treats, how long there’s been a shortage, the reason for the shortage and manufacturer contact information.
Again, the entries are basic but provide the most important information: the clinical area the drug treats, how long there’s been a shortage, the reason for the shortage and manufacturer contact information.
The same information is provided for drug shortages that have been resolved, useful if you need to follow up on a past shortage (or, in the case of the screenshot below, point out that they either have a typo on their dates or have begun a new-fangled way of writing them).
For discontinued drugs, a brief (usually vague) reason is provided. If your Spidey sense tingles when skimming one of these, it may be worth finding out what’s meant by a “business decision.” While it may be a run-of-the-mill, business-as-usual reason, having the app on your phone enables you periodically skim through the list and note anything surprising or unusual that’s worth checking out.