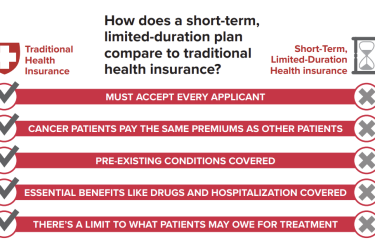
President Donald Trump has spoken about the cost of drugs frequently. Though not always clearly and consistently, he has called for government negotiation of prices. Drug prices also have caught Congress’s eye, although we’re not quite sure what (if anything) legislators are willing to do about it.
President Donald Trump has spoken about the cost of drugs frequently. Though not always clearly and consistently, he has called for government negotiation of prices. Drug prices also have caught Congress’s eye, although we’re not quite sure what (if anything) legislators are willing to do about it.
But patients are fed up, as David Mitchell, founder of Patients for Affordable Drugs reminded us last week during a Health Journalism 2017 session in Orlando.
“People are angry, people are upset, people are hurting. And it shows up in the stories we are collecting,” said Mitchell, whose new organization has received 5,000 patient stories in seven weeks. Mitchell, who has multiple myeloma, also noted that, despite an abundance of patient advocacy groups, their voices on costs often are not heard. Most of the largest advocacy group receive some drug company funding, as a recent study in NEJM noted. With very few exceptions (notably the MS Society), patient groups are not advocating on prices even though, as Mitchell noted, they do other good work that helps patients.
Mitchell, along with Peter Bach, director of the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, and Mark McClellan, director of the Margolis Center for Health Policy at Duke University, addressed the topic at the conference panel “Drug Costs: Intersection of Business, Public Health and Policy.” Jayne O’Donnell of USA Today moderated.
On several points, the panelists had broad agreement – especially the belief that the system addresses neither the needs of Medicaid and Medicare patients nor the taxpayers who pay their drug bills.
“I’ve sort of given up on competition,” said Bach. “We have higher prices than anywhere else and poorer access.”
The panelists saw some areas in which Congress could find bipartisan consensus or in which the FDA can take regulatory steps on its own, particularly regarding the lack of competition with generic drugs. They thought drug discount coupons, although beneficial for individual patients, serve to disguise actual prices and blunt calls to action. Bach called them “corrosive.”
There was discussion about whether there should be more transparency in how pharmacy benefit managers (PBMs) operate, and whether or not the discounts they negotiate are passed on. Some research suggests transparency might thwart discount negotiations if all players can see the deals everyone else is cutting, McClellan noted.
There was less consensus on drug importation and Medicare drug negotiations. Panelists were split on the value of allowing importation or reimportation of drugs from foreign countries. McClellan is among several former FDA commissioners (from both Democrat and Republican administrations) who have safety concerns about overseas supply chains. Mitchell favors allowing importation from certain countries such as Canada, arguing that the risk of importing drugs from a developed country with a good regulatory framework is small compared with the benefit of enabling patients to obtain medicines they otherwise could not afford.
But McClellan is skeptical that allowing Medicare to negotiate drug prices would make much difference – a view the Congressional Budget Office supports. Medicare is a huge payer of drugs but it doesn’t have a formulary like that of the Veterans Administration or private insurers, so it is obligated to cover a broader array of drugs. That means it doesn’t have all that much clout other than its “soapbox,” he said. (See this Kaiser Family Foundation issue brief on Medicare drug policy.)
The other panelists noted that there are ways to address Medicare drug policy more broadly that could give the agency more power to pick and choose. Mitchell said a formulary would be acceptable, as long as there are exemptions when a patient truly needs a drug that is off formulary.
Both McClellan and Bach said they strongly favored finding ways to price drugs based on their value. One idea in circulation is to charge different prices for a drug depending on the indication for which it is prescribed. “The idea is to use a structure to find prices for drugs based on value, which includes effectiveness and toxicity,” said Bach. McClellan said drug prices should reflect what we care about – “the outcome.” Pharmaceutical pricing should be part of the overall push for value that is shaping reform in the rest of the health care system.
But Mitchell was impatient with the idea of “paying for value.” If a drug has no value, it shouldn’t be on the market in the first place. “If they don’t work, don’t give it to me,” he said. “I’ll die if it doesn’t work. This isn’t theoretical.”
See the tweets from this panel for more tips.
Health Journalism 2017
- Cinematic techniques can add pop to stories, says Pulitzer winner
- panel explored humanizing medicine in a high-tech world
- #AHCJ17 panelists wrestle with the unintended consequences of complying with MACRA
- #AHCJ17 panel addresses the cycle of toxic stress in young children
- What’s next for drug costs? #AHCJ17 hears from the experts – including a patient
- Feeding hungry seniors and much more, one knock on the door at a time
- Social workers can help bridge crisis and care, say panelists at #AHCJ17
- Freelancers weigh in on what it takes to successfully write a book at #AHCJ17
- Public health experts discuss ‘infectious nature’ of violence
- #AHCJ17 panels to address importance of social determinants
- Is value-based pricing doomed? #AHCJ17 session to address this question
- Panel to examine MACRA, the physician payment overhaul law
- Panel to look at the economics of health disparities
- Journalists to learn about security, privacy tools and practices